Recent Advances in the Battle Against COVID-19
The world breathed a collective sigh of relief at Pfizer and BioNTech’s announcement of a COVID-19 vaccine candidate passing phase 3 trials. In response, the markets roared on November 9, 2020 with a massive rotation from technology/stay-at-home-stocks to small caps, energy, and industrials. It was a great morning for the stock market record books.
More positive news in the fight against COVID-19 came after the November 9, 2020 market close. That evening, Eli Lilly & Co’s (NYSE: LLY) antibody treatment received emergency F.D.A. approval. The authorization applies only to people newly infected with the SARS-CoV-2 virus, and the agency said it should not be used in hospitalized patients. The company indicated its treatment, called bamlanivimab, should be administered as soon as possible after a positive test and within 10 days of developing symptoms.
Eli Lilly & Co. Chairman and Chief Executive Officer David Ricks discussed his company’s COVID-19 antibody therapy, which could reduce hospitalizations of patients with underlying conditions and in high risk groups. However, according to The New York Times, Eli Lilly said it expects to have enough doses to treat one million people by the end of the year. That means, even in the best-case scenario, there won’t be nearly enough doses to curb a virus that is now infecting an average of over 116,000 people a day in the United States.
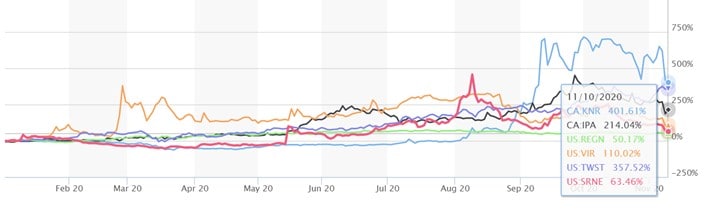
Eli Lilly, Pfizer, and BioNTech aren’t the only firms investors should monitor. Other companies working on antibody therapies that we believe are going to be important in the fight against COVID-19 include ImmunoPrecise Antibodies (TSXV: IPA, OTC: IPATF), Regeneron Pharmaceuticals (NASDAQ: REGN), Vir Biotechnology (NASDAQ: VIR), Twist Bioscience (NASDAQ: TWST), and Sorrento Therapeutics (NASDAQ: SRNE). We highlight their year-to-date stock returns below and note all these stocks have been volatile with news-flow.
A Light in the Darkness – Examining the Vaccine Solution
We, like most people, are hopeful that this Pfizer/BioNTech vaccine candidate and other advances like antibody therapies will eventually help us return to the normal activities that we’ve paused since March 2020. That said, while recent market moves signal that COVID-19 risks have passed, we believe that the virus could continue to impact the world for some time to come. Although we remain optimistic regarding recent therapeutic and vaccine advances, we recognize that the COVID-19 healthcare toolkit will likely require more tools; it will also need rapid testing kits, hand sanitizers, social distancing, and virus detection systems.
Echoing our view about COVID-19’s continued risks, Mr. Brian Bloom, co-founder, chairman and chief executive officer at Bloom Burton & Co. (a healthcare sector focused investment bank) suggested, “This is not the light at the end of the tunnel … this is a light in the darkness”. Taking Mr. Bloom’s statement further, let’s assume (and hope) that the Pfizer/BioNTech vaccine candidate is the global cure for COVID-19. Significant challenges remain including:
- Manufacturing the vaccine, en masse. Merck CEO Ken Frazier stated that a vaccine has never been used on 7.5 billion people before;
- Vaccine distribution, especially in countries where transportation, communication, electrical, and refrigeration infrastructure is not strong;
- Ultra-nationalism, where governments take what’s developed in their countries and vaccinate their citizens first;
- Vaccine affordability;
- Resurgence of COVID-19, its resilience in the United States, and additional strains in Europe;
- A June 2020 survey of 13,426 people across 19 countries indicated that 71.5% of participants would be very or somewhat likely to take a COVID-19 vaccine, which could prolong the pandemic and continue to strain health systems due to the balance of participants likely declining the vaccine.
As well, the Pfizer press release states:
- Clinical trial to continue through to final analysis at 164 confirmed cases in order to collect further data and characterize the vaccine candidate’s performance against other study endpoints.
- Based on current projections we expect to produce globally up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021.
- Pfizer and BioNTech plan to submit data from the full Phase 3 trial for scientific peer-review publication.
So, even if peers validate Pfizer/BioNTech’s data after the clinical trial ends, it could take years before there are enough doses for every person in the world. Echoing this point, on November 9, 2020 President-elect Joe Biden lauded the news of Pfizer’s progress on a COVID-19 vaccine but urged Americans to be cautious because widespread vaccination is still months away. Biden said the news does not mean there is a cure, and he urged patience while setting expectations that the timetable for any potential vaccine had not changed. It could be well into 2021 before there is widespread vaccination.
Detection is the Bridge to the Cure and Controlling Case Loads For Therapies
We believe that until any effective vaccine can cure COVID-19 (as well other emergent viruses as and when surface, now that the world has awoken to this threat), virus detection and contact tracing is the best intermediary method to protect people and stem the spread. Kontrol Energy (CSE:KNR, OTCQB:KNRLF), a Sophic Capital client and an emerging leader in the Smart Buildings industry, has developed CSA standards approved technology called BioCloud® that detects COVID-19 in real-time and issues alerts. In basic terms, think of BioCloud as a smoke detector-like device that alerts to the presence of COVID-19 rather than fire. While Kontrol’s BioCloud cannot prevent infected people from passing the virus onto others, it can limit the number of people infected through early detection and can thus play a significant role in initiating early contact tracing. Compare this to an infected person testing positive for symptoms 3 weeks after contracting the virus, and then trying to trace everyone who was exposed to the infected person during those 3 weeks.
Coming Up: A Deeper Look at Kontrol’s BioCloud Solution, and Some Data Points Relevant to Investors
In our next report, we look at Kontrol’s BioCloud’s potential applications in multiple industries and some possible competitors. We also look at some data points pertaining to Kontrol’s business, which are of relevance to investors.